Committee for the Protection of Human Subjects
Policy & Procedures
Policy & Procedures (Revised November 2025)
What Type of Review Does Your Research Need?
Classifications of Research Involving Human Subjects
Exemption Category A - Frequently Asked Questions
Application Procedures - Detailed
CPHS Checklist for Researchers and Reviewers
CPHS Informed Consent Document Template
Review Timeline
Expedited reviews require five business days with the Committee and time for administrative processing.
Full reviews require ten business days with the Committee and time for administrative processing.
Protocols are reviewed continuously throughout the fall and spring semesters. Only externally funded protocols are reviewed during academic calendar summer and winter breaks.
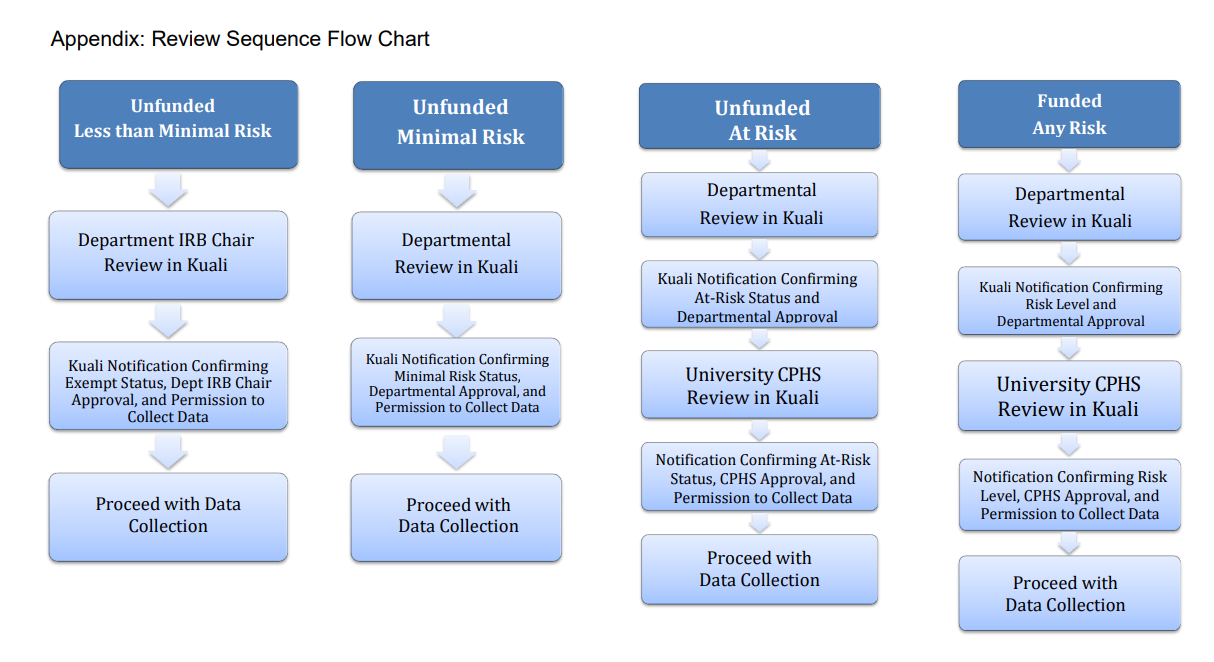
Protocol Review Process Flowchart
NOTE: The CPHS has the responsibility for reviewing and the authority to approve,
require modification, or disapprove all research and related activities involving
human subjects under the auspices of California State University, Fresno, including
previously approved activities. This includes exempt and minimal risk protocols.